My Experience on Paper Presentation At The 1st International Conference in Advances on Science |ChemFam #30|
Greetings to all my dear fellow Hivians! This post is not going to be a solely chemophilic topic related but more of the work I have been putting into a research project and also about my experience on delivering my paper presentation at the first International Conference on Advances in Science (Hybrid Mode) held at the Gargaon College.

I have been asked by my mentor to give a paper presentation at the conference around a week prior to the auspicious event. I have been working on my research project with my friend Jilhaz Ahmed who is extremely helpful. I also have been informed that we will be the only Master degree students participating on that event. We were excited yet nervous as well hearing that we will be the sole participant as students. Nevertheless we started our work for the paper hoping to get some exposure in front of well established professors and Researchers. We also had our sessional examinations during that week, so we had to give time for our exam preparation too.
As I have already posted about the topic I have been working currently that is the Acceptorless Dehydrogenation. My presentation was based on the same subject. I have prepared my presentation entitled “Nanoporous Montmorillonite Clay Stabilized Copper Nanoparticles: Efficient and Reusable Catalyst for Acceptorless Dehydrogenation of Alcohols to Carboxylic Acids”.

I won’t lie but I did not prepare much for the presentation. To be honest, I did not find time for it. I have reached the location where the conference was going to happen at around 11 am on 6th May. As it was organised in a hybrid mode so both online and offline presentations were meant to be presented by professors and researchers. When I reached the hall, the online presentations were happening and I enjoyed listening to them. One of the paper presenter presented on AI roles on smart learning and I must say it was a great one. My mentor also presented his work on Organometallic Ruthenium catalyst.
My presentation was scheduled at 2.30 pm. So I went to have the lunch. I came back and the time came for my presentation. I was invited to the dais, with a bit of nervousness and excitement I started my presentation. I greeted everyone presented there and started with a brief discussion about two basic topics covering my paper - Clay and Acceptorless Dehydrogenation. I gave a rough idea of clay and their structure and properties. Since my catalyst was supported on a special kind of clay which is montmorillonite belonging to the smectite group. So I had to talk about the clay and the reason for using the clay for chemical conversion that I was looking for. Then I also talked about the acceptorless dehydrogenation, it’s working and the advantages over conventional oxidations of organic compounds. You can read my article on acceptorless dehydrogenation for better understanding. I will also be writing on Clays, specifically about montmorillonite clay in the future days to come.
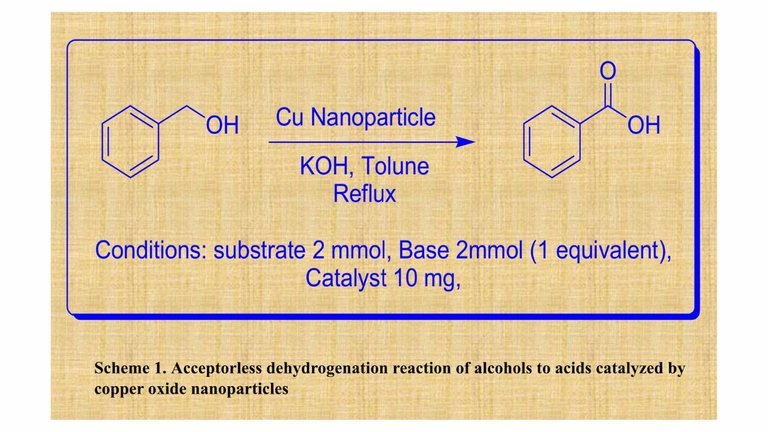
For summarising my work, I have developed a copper nanoparticle catalyst supported on the surface of montmorillonite clay to facilitate the conversion of alcohols to carboxylic acids. Firstly, I have treated the clay with 4 molar phosphoric acid. I have used 2 grams of clay with 100 ml of phosphoric acid and dispersed them really well in a round bottom (RB) flask with the help of a magnetic stirrer. I have allowed the RB to reflux for about 30 minutes. I have prepared such acid treated clay for different durations such as for 1 hour, 1.5 hours, 2 hours, 2.5 hours, 3 hours, 3.5 hours and 4 hours. Then I have centrifuged the acid acid treated clay and allowed to dry for a day at 60°C. The dried clay was grounded using a mortar and pestle.
The dried clay sample was less in amount than the initial clay I took. I have taken 0.5 grams of the acid treated clay and added some distilled water and (CH3COO)2Cu.H2O was added to it. The reaction was allowed to proceed for 24 hours. Next day, I have reduced the solution using NaBH4. The colour of the solution turned black after adding the reducing agent. I have filtered the solution and allowed it to dry for a day at 60°C. After drying it was grounded and that is our copper nanoparticle catalyst. The catalyst is reusable and it is efficient for conversion of alcohols to carboxylic acids.
Our main goal was to convert benzyl alcohol to benzoic acid. We have used KOH as our base and toluene as our solvent and most importantly the catalyst we have prepared. The final product as well as the catalyst was studied by different techniques such as 1H NMR, 13C NMR, PXRD, SEM, FTIR, HRMS.

I have received a participation certificate for presenting my paper and also received an abstract for the same. It was a great experience for me to present my work in such a huge event in front of well established researchers. We were the sole master degree students participating on that event and we really enjoyed every moment.
The conference went really well and it was a success for the organising committee. On the contrary, it was a great opportunity for me to present my work on such a big stage. I shall see you soon with a new post. Till then stay happy, stay safe.
The Accidental Cure for Cancer: Cisplatin |ChemFam #29|
Acceptorless Dehydrogenation and Related Transformations |ChemFam #28|
Thermophysical Properties of Natural Gas-I |ChemFam #27|
Sources and Process Overview of Natural Gas |ChemFam #26|
Recovery, Upgradation and Purification of Helium in Natural Gas |ChemFam #25|
Trace Components in Natural Gas System |ChemFam #24|
Sulphur Recovery in Natural Gas System-II |ChemFam #23|
Sulphur Recovery in Natural Gas System-I |ChemFam #22|
Nitrogen Removal in Natural Gas System-II |ChemFam #21|
Nitrogen Removal in Natural Gas System-I |ChemFam #20|
PS All the images belongs to me and are not permitted to use without my authorisation.


Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.